Use of Single Use Technologies (SUT) for sterile Dosage form processing and filling operations
Mr. Chandan Kumar Sah, Senior Manager- Quality/Technical, Biotechnologist
Introduction:
Pharmaceutical drug products are produced to be efficacious, however, the presence of microorganisms or microbial by products in these products may have adverse effects on their efficacy. Contamination of aseptically filled biotechnological products is costly and can pose serious harm to the patient. Sterile dosage forms are those which are free from any microorganism, dust, fibers, and foreign particles and should be isotonic. Parenteral preparation as name suggest (Par-enteral) are those which are administered other than enteral routes. Enteral route involves esophagus, stomach, intestines but parenteral route bypasses all these. Sterile dosage forms include parenteral preparation and ophthalmic preparation. Parenteral preparations include injections, Transfusions fluids, sterile suspensions, sterile solids, sterile solutions or emulsions. Ophthalmic preparations include eye drops, eye lotions, eye ointments, eye gels, eye suspensions, contact lens solutions.
Ideal properties of sterile dosage forms:
- Sterility: Sterile preparation shall be free from all type of microorganism. Ophthalmic formulation must be especially free from gram negative bacteria.
- Free from pyrogen: Sterile formulation must be free from pyrogens and toxins. These products must pass pyrogen test.
- Free from foreign particle: these products must be free from foreign particles, dust, fibers and must pass clarity test.
To achieve this properties Ami polymer private limited play vital role in supplying critical process consumable that is single use assemblies to parenteral formulation industries. Single- use, sterile disposable technologies (sometimes referred to as bio disposable technologies) are available in many different formats and confer various advantages for pharmaceutical manufacturers. Single use disposable technologies are generally manufactured from plastic/elastomeric polymers involving process of injection moulding, extruding and blow moulding. The assembly of components should be undertaken in an ISO 14644 class 5 to ISO class 7 clean room. Once assembled the items are sterilized using gamma irradiation.
Single-use items are typically sterilized using gamma rays. Gamma irradiations kills bacteria, where there is sufficient energy, at the molecular level by breaking down bacterial DNA and inhibiting bacterial division. The sterilization cycles are designed to achieve a sterility Assurance level 10-6.
There are several types of single use systems. This article examines some examples that are applicable to biotechnological aseptic processing: aseptic connections, disposable product holding systems and bio container bags.
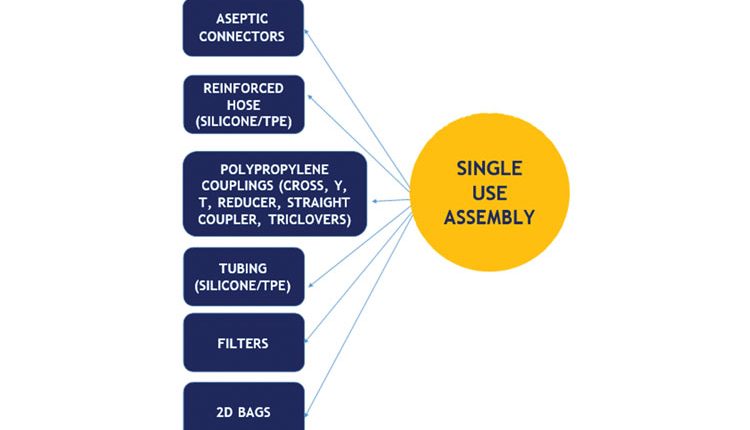
Common Single Use Process Components.
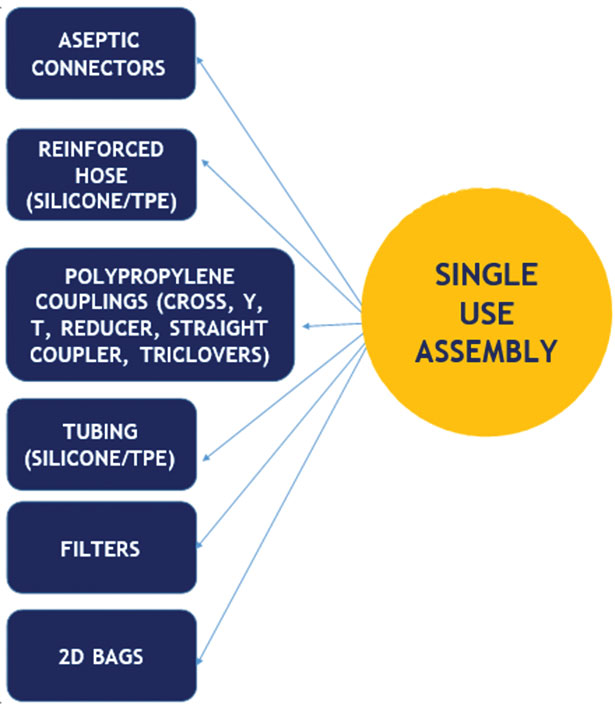
Application of Single use technology (SUT) for biotechnological aseptic processing:
Aseptic connection:
A critical clean room step is the aseptic connection, especially for aseptically filled products. An aseptic connection allows fluid to be passed from one vessel to another in a way that does not introduce microbial contamination. Ami polymer private limited provide one step solution by providing high quality customize single use manifold which allow the operator to do connection in a very easy mode and maintained the aseptic connection.
Cost Savings:
Cost savings are bound into process efficiencies. Although the initial cost of purchasing single use assemblies is generally greater than the recycling of stainless steel components, the benefits of a faster turnaround, which potentially allows an organization to produce faster and move between different product streams more quickly, deliver longer-term, cost savings. Perhaps the greatest cost saving of all depending on the valve of the product, is the elimination of contamination events, which will lead to batch rejection and process downtime.
Process efficiencies:
Single use technologies present an opportunity for process efficiencies, several significant advantages over standard reusable stainless steel system, particularly in reducing process downtime and removing the need to clean and sterilize items. This eliminates the need to turnaround equipment thereby presenting opportunities to save on such factors as energy, waste disposal, cleaning chemicals used and labor.
Cross Contamination:
Cross- contamination in pharmaceutical processing can be a sterility assurance issue or a matter of product adulteration. Cross contamination can arise through the recycling of equipment where product residues are not adequately removed. Disposable components are single use only and therefore not used for repeated operations, eliminating the chance of cross contamination or product carry over between process runs.
As a conclusion of all this Ami polymer private limited offers wide range of gamma irradiated single use assemblies for various critical applications in biopharmaceuticals industries to support the campaign of Large COVID vaccine drive of India. Our Single use assemblies are used to manufacture varieties of life saving drugs {Covishiled vaccine, Covaxin, J&J Vaccine (Trial Ongoing), Corbevax (Trial Ongoing). These are range from simple tubing with connector to complex manifold with several connections. All the assemblies are manufactured and packed in Class 7 certified clean room while critical components are assembled in class 5 certified clean room. Most of the key components used in this Single use assemblies are in-house manufactured and endure with high quality standard.
Website: www.amipolymer.com